Abstract
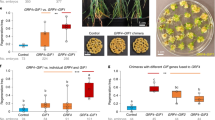
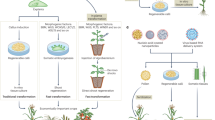
Although great progress has been achieved regarding wheat genetic transformation technology in the past decade1,2,3, genotype dependency, the most impactful factor in wheat genetic transformation, currently limits the capacity for wheat improvement by transgenic integration and genome-editing approaches. The application of regeneration-related genes during in vitro culture could potentially contribute to enhancement of plant transformation efficiency4,5,6,7,8,9,10,11. In the present study, we found that overexpression of the wheat gene TaWOX5 from the WUSCHEL family dramatically increases transformation efficiency with less genotype dependency than other methods. The expression of TaWOX5 in wheat calli prohibited neither shoot differentiation nor root development. Moreover, successfully transformed transgenic wheat plants can clearly be recognized based on a visible botanic phenotype, relatively wider flag leaves. Application of TaWOX5 improved wheat immature embryo transformation and regeneration. The use of TaWOX5 in improvement of transformation efficiency also showed promising results in Triticum monococcum, triticale, rye, barley and maize.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Accession numbers and gene names are available from the phylogenetic tree in Extended Data Fig. 1. The accession numbers of genes identified in this study are available in Supplementary Table 1, and their sequences are provided in the Supplementary sequence file. The accession number of pWMB111 is MZ458107. Raw data for experiments are available in Supplementary Tables 2 and 3. Transgenic lines and plasmids generated are available from the corresponding authors on request. Source data are provided with this paper.
Change history
30 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41477-022-01173-3
References
Ishida, Y. Tsunashima, M. Hiei, Y. & Komari, T. in Agrobacterium Protocols Vol. 1 (ed. Wang, K.) 189–198 (Springer, 2015).
Richardson, T., Thistleton, J., Higgins, T. J., Howitt, C. & Ayliffe, M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell Tissue Organ Cult. 119, 647–659 (2014).
Wang, K., Liu, H. Y., Du, L. P. & Ye, X. G. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. J. 15, 614–623 (2017).
Zakizadeh, H., Stummann, B. M., Lutken, H. & Muller, R. Isolation and characterization of four somatic embryogenesis receptor-like kinase (RhSERK) genes from miniature potted rose (Rosa hybrida cv. Linda). Plant Cell Tissue Organ Cult. 101, 331–338 (2010).
Lotan, T. et al. Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93, 1195–1205 (1998).
Stone, S. L. et al. LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc. Natl Acad. Sci. USA 98, 11806–11811 (2001).
Nishimura, A. et al. Isolation of a rice regeneration quantitative trait loci gene and its application to transformation systems. Proc. Natl Acad. Sci. USA 102, 11940–11944 (2005).
Boutilier, K. et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14, 1737–1749 (2002).
Bouchabke-Coussa, O. et al. Wuschel overexpression promotes somatic embryogenesis and induces organogenesis in cotton (Gossypium hirsutum L.) tissues cultured in vitro. Plant Cell Rep. 32, 675–686 (2013).
Zuo, J. R., Niu, Q. W., Frugis, G. & Chua, N. H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 30, 349–359 (2002).
Lowe, K. et al. Morphogenic regulators baby boom and wuschel improve monocot transformation. Plant Cell 28, 1998–2015 (2016).
Lowe, K. et al. Rapid genotype ‘independent’ Zea mays L. (maize) transformation via direct somatic embryogenesis.In Vitro Cell Dev. Biol. Plant 54, 240–252 (2018).
Gao, H. R. et al. Superior field performance of waxy corn engineered using CRISPR–Cas9. Nat. Biotechnol. 38, 579–581 (2020).
Hoerster, G. et al. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation.In Vitro Cell Dev. Biol. Plant 56, 265–279 (2020).
Debernardi, J. M. et al. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38, 1274–1279 (2020).
Su, Y. H. et al. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 59, 448–460 (2009).
van der Graaff, E., Laux, T. & Rensing, S. A. The WUS homeobox-containing (WOX) protein family. Genome Biol. 10, 248 (2009).
Sarkar, A. K. et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814 (2007).
Zhang, W. et al. Regeneration capacity evaluation of some largely popularized wheat varieties in China. Acta Agron. Sin. 44, 208–217 (2018).
Riaz, B. et al. Overexpression of maize ZmC1 and ZmR transcription factors in wheat regulates anthocyanin biosynthesis in a tissue-specific manner. Int. J. Mol. Sci. 20, 5806 (2019).
Wang, X. M. et al. Effects of environmental temperature on the regeneration frequency of the immature embryos of wheat (Triticum aestivum L.). J. Integr. Agric. 13, 722–732 (2014).
Tao, L. L. et al. Improvement of plant regeneration from immature embryos of wheat infected by Agrobacterium tumefaciens. Agric. Sci. China 10, 317–326 (2011).
Liu, H. Y. et al. Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. J. Exp. Bot. 71, 1337–1349 (2021).
Bartlett, J. G., Alves, S. C., Smedley, M., Snape, J. W. & Harwood, W. A. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 4, 22 (2008).
Ishida, Y., Hiei, Y. & Komari, T. Agrobacterium-mediated transformation of maize. Nat. Protoc. 2, 1614–1621 (2007).
Sambrook, J. Russell, D. W. Molecular Cloning, A Laboratory Manual 3rd ed. Chapter 1, 34–36 (Cold Spring Harbor Laboratory Press, 2001).
Paternò, A. et al. In-house validation and comparison of two wheat (Triticum aestivum) taxon-specific real-time PCR methods for GMO quantification supported by droplet digital PCR. Food Anal. Methods 11, 1281–1290 (2018).
Acknowledgements
We thank W. Xiao at Saint Louis University, USA and H. Li at the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences for critical revisions of this manuscript. This research was financially supported by grants from the National Natural Science Foundation of China (no. 31971946) to K.W., the Science and Technology Department of Ningxia in China (no. 2019BBF02020) to X.Y. and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (nos. S2021ZD03 and 2060302-2-19) to K.W. and X.Y..
Author information
Authors and Affiliations
Contributions
K.W. contributed to funding acquisition, experimental design, vector construction, wheat and barley transformation, data analysis and manuscript writing. L.S. contributed to gene identification, vector construction and transgenic detection. X.L. performed medium modification and wheat transformation. P.Z. was involved in gene identification and sequence analysis. W.W. was involved in barley transformation and manuscript writing. J.L. performed transformation of T. monococcum and rye. Y.C. performed transformation of triticale. Y.H. performed transformation of maize. C.Y. contributed to vector construction. L.D. contributed material management and medium preparation. Y.I. contributed to experimental design, wheat transformation and manuscript editing. X.Y. conceived the study, supervised experiments, conducted formal analysis and contributed to project administration, funding acquisition and manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
K.W., X.Y. and L.D. (all ICS-CAAS) are co-inventors in Chinese patent application no. ZL201710422896.6. X.Y., K.W., L.S. and L.D. (all ICS-CAAS) and Y.I. and C.Y. (both JT) are co-inventors in international patent application no. PCT/CN2018/090239, in which ICS-CAAS and JT had shared ownership; the share of the latter was assigned to Kaneka Corporation, a Japanese chemical company, on 29 January 2021. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Sadiye Hayta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Phylogenetic relationships among TaWOX5 and TaWUS proteins from wheat, and WOX proteins from Arabidopsis.
The phylogenetic tree was constructed based on the sequences of TaWOX5 and TaWUS proteins in wheat and WOX proteins in Arabidopsis in MEGA X using the neighbor-joining approach with 1,000 bootstrap replicates. Scale plate and legend in left display tree scale and bootstrap value. AtWUS: NP_565429; AtWOX1: NP_188428; AtWOX2: NP_200742; AtWOX3: NP_180429; AtWOX4: NP_175145; AtWOX5: NP_187735; AtWOX6: NP_565263; AtWOX7: NP_196196; AtWOX8: NP_199410; AtWOX9: NP_180944; AtWOX10: NP_173494; AtWOX11: NP_187016; AtWOX12: NP_197283; AtWOX13: NP_195280; AtWOX14: NP_173493. TaWOX5: MN412513; TaWUS-A: MW452946; TaWUS-B: MW452947; TaWUS-D: MW452945.
Extended Data Fig. 2 Shoot regeneration of the immature embryos of different wheat genotypes promoted by the TaWOX5 gene.
a: Shoot regeneration of the wheat embryos transformed with control vectors. b: Shoot regeneration of the wheat embryos transformed with TaWOX5 gene containing vector. The calli on plates were some overcrowding.
Extended Data Fig. 3 Detection of transgenic wheat plants by QuickStix Kit and PCR.
a: QuickStix Kit assay for the Bar protein; 1-21: transgenic plants; 22: wild-type Fielder. b: PCR detection for Bar gene, this testing experiment being repeated at least three times with similar results; 1: plasmid of TaWOX5 vector; 2: wild-type Fielder; 3-24: transgenic plants.
Extended Data Fig. 4
Comparison of the transient infection efficiency of different wheat varieties by expressing anthocyanin biosynthesis genes ZmR and ZmC1 as visible markers.
Extended Data Fig. 5 Normal growth of the regeneration shoots and roots derived from a transformed immature embryo of Fielder using the TaWOX5 gene in three experimental replicates.
a: The growth status of regeneration shoots; b: the growth status of the transgenic plants with healthy shoots and roots.
Extended Data Fig. 6
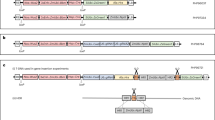
The plasmids map of pWMB111-TaWOX5 and TaWOX5- SpCas9-TaQ.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and the sequences of TaWOX5 and TaWUS used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Wang, K., Shi, L., Liang, X. et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 8, 110–117 (2022). https://doi.org/10.1038/s41477-021-01085-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-01085-8
This article is cited by
-
Inadequate lysine content of wheat endosperm proteins - possibility of correcting it by CRISPR-Cas system of genome editing
Journal of Plant Biochemistry and Biotechnology (2024)
-
Identification of WUSCHEL-related homeobox gene and truncated small peptides in transformation efficiency improvement in Eucalyptus
BMC Plant Biology (2023)
-
Effect of a suitable treatment period on the genetic transformation efficiency of the plant leaf disc method
Plant Methods (2023)
-
Single-cell resolution analysis reveals the preparation for reprogramming the fate of stem cell niche in cotton lateral meristem
Genome Biology (2023)
-
An E2-E3 pair contributes to seed size control in grain crops
Nature Communications (2023)