Abstract
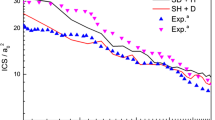
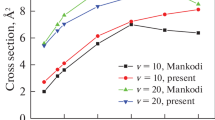
The quantum dynamics calculations of O+ + H2 (vi = 0, ji = 0) → OH+ + H reaction have been performed using a Chebyshev wave packet method on a new potential energy surface constructed by Song et al. [Y.Z. Song et al., Chin. Phys. B 24, 063101 (2015)]. The reaction probabilities of partial wave J = 0–80 are calculated explicitly with the centrifugal sudden (CS) and Coriolis coupling (CC) calculations. The comparison between the CC and the corresponding CS results indicates that neglecting the Coriolis coupling will lead to the underestimation of the reaction probability. The comparison of theoretical integral cross sections (ICS) with the experimental results in the collision energy range of 0.0–1.0 eV indicates that the calculated values in this work agree well with the experimental data and other theoretical results, showing the reasonability of PES and dynamic calculations. The obtained ICSs are used to calculate the rate constants in the temperature range of 0–2000 K, and the results at room temperature reasonably agree with the experimental data.
Graphical abstract

Similar content being viewed by others
References
W.W. Duley, D.A. Williams, Interstellar Chemistry (Academic, New York, 1984)
C.Y. Ng, J. Phys. Chem. A 106, 5953 (2002)
D. Smith, N.G. Adams, T.M. Miller, J. Chem. Phys. 69, 308 (1978)
X. Li, Y.L. Huang, G.D. Flesch, C.Y. Ng, J. Chem. Phys. 106, 1373 (1997)
J.D. Burley, K.M. Ervin, P.B. Armentrout, Int. J. Mass Spectrom. Ion Process. 80, 153 (1987)
A.A. Viggiano, J.M. Van Doren, R.A. Morris, J.S. Williamson, P.L. Mundis, J.F. Paulson, C.E. Dateo, J. Chem. Phys. 95, 8120 (1991)
L.S. Sunderlin, P.B. Armentrout, Chem. Phys. Lett. 167, 188 (1990)
K.T. Gillen, B.H. Mahan, J.S. Winn, J. Chem. Phys. 58, 5373 (1973)
K.T. Gillen, B.H. Mahan, J.S. Winn, J. Chem. Phys. 59, 6380 (1973)
R. Martínez, J. Millán, M. González, J. Chem. Phys. 120, 4705 (2004)
R. Martínez, J.D. Sierra, M. González, J. Chem. Phys. 123, 174312 (2005)
R. Martínez, J.D. Sierra, S.K. Gray, M. González, J. Chem. Phys. 125, 164305 (2006)
R. Martínez, J.M. Lucas, X. Giménez, A. Aguilar, M. González, J. Chem. Phys. 124, 144301 (2006)
J. Kłos, N. Bulut, S. Akpinar, Chem. Phys. Lett. 532, 22 (2012)
W. Xu, W. Li, S. Lv, H. Zhai, Z. Duan, P. Zhang, J. Phys. Chem. A 116, 10882 (2012)
N. Bulut, J.F. Castillo, P.G. Jambrina, J. Klos, O. Roncero, F.J. Aoiz, L. Banñares, J. Phys. Chem. A 119, 101951 (2015)
Y.Z. Song, Y. Zhang, L.L. Zhang, S.B. Gao, Q.T. Meng, Chin. Phys. B 24, 063101 (2015)
H.J. Werner, P.J. Knowles, J. Chem. Phys. 89, 5803 (1988)
R.A. Kendall, T.H. Dunning Jr., R.J. Harrison, J. Chem. Phys. 96, 6796 (1992)
T.H. Dunning Jr., J. Chem. Phys. 90, 1007 (1989)
J.Z.H. Zhang, Theory and Application of Quantum Molecular Dynamics (World Scientific, Singapore, 1999)
S.Y. Lin, H. Guo, J. Chem. Phys. 119, 11602 (2003)
S.B. Gao, J. Zhang, Y.Z. Song, Q.T. Meng, Eur. Phys. J. D 69, 111 (2015)
S.B. Gao, L.L. Zhang, Y.Z. Song, Q.T. Meng, Chem. Phys. Lett. 651, 233 (2016)
W. Wei, S.B. Gao, Z.P. Sun, Y.Z. Song, Q.T. Meng, Chin. Phys. B 23, 073101 (2014)
V.A. Mandelshtam, H.S. Taylor, J. Chem. Phys. 102, 7390 (1995)
V.A. Mandelshtam, H.S. Taylor, J. Chem. Phys. 103, 2903 (1995)
H. Tal-Ezer, R. Kosloff, J. Chem. Phys. 81, 3967 (1984)
D.H. Zhang, J.Z.H. Zhang, J. Chem. Phys. 101, 1146 (1994)
A.J.H.M. Meijer, E.M. Goldfield, S.K. Gray, G.G. Balint-Kurti, Chem. Phys. Lett. 293, 270 (1998)
S.C. Althorpe, J. Chem. Phys. 114, 1601 (2001)
A. Messiah, Quantum Mechanics (John Wiley & Sons, Inc., New York, 1968)
S.Y. Lin, H. Guo, J. Phys. Chem. A 108, 2141 (2004)
S.Y. Lin, H. Guo, J. Chem. Phys. 124, 031101 (2006)
T.E. Carroll, E.M. Goldfield, J. Phys. Chem. A 105, 2251 (2001)
A.J.H.M. Meijer, E.M. Goldfield, J. Chem. Phys. 110, 870 (1999)
T.S. Chu, K.L. Han, Phys. Chem. Chem. Phys. 10, 2431 (2008)
J.M. Bowman, J. Phys. Chem. 95, 4960 (1991)
S.K. Gray, E.M. Goldfield, G.C. Schatz, G.G. Balint-Kurti, Phys. Chem. Chem. Phys. 1, 1141 (1999)
D.C. Clary, Mol. Phys. 53, 3 (1984)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, F., Wang, X., Zhao, W. et al. Quantum dynamics calculations for O+ + H2 (vi = 0, ji = 0) → OH+ + H ion–molecule reaction on a new potential energy surface. Eur. Phys. J. D 72, 224 (2018). https://doi.org/10.1140/epjd/e2018-90144-5
Received:
Revised:
Published:
DOI: https://doi.org/10.1140/epjd/e2018-90144-5